How To Do Molecular Orbital Diagrams
Molecular orbital diagrams Orbital molecular he2 o2 be2 bond bonding paramagnetic diamagnetic orbitals electrons diatomic molecules chem unpaired sigma antibonding vertical nitrogen li Inorganic chemistry
Introductory Chemistry 1.0 | FlatWorld
Orbital khan understanding 4.9: molecular orbitals 37+ molecular orbital geometry image
Molecular orbital theory (mot)
Introductory chemistry 1.0Day 6: molecular orbitals; lewis structures – chemistry 109, fall 2020 Solved 7. consider the molecular orbital diagram below. (a)Orbital molecular orbitals bonding octahedral ml h20 molecule consider.
Molecular orbitals atomic orbital bonding mot energies sigma chem libretexts npElectron orbitals electrons quantum numbers chemistry electronic structure atoms introductory model orbital figure atomic number arrangement energy ball libretexts principal Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level electron cl2 libretexts second delocalized homonuclear rowOrbital molecular mo o2 diagram theory orbitals bond oxygen order paramagnetic configuration energy electrons unpaired diagrams two lone draw which.

He2 2+ molecular orbital diagram
9.8: second-row diatomic moleculesMolecular orbitals diatomic orbital molecules valence electrons electron bonding paramagnetic delocalized libretexts principles heteronuclear chem pageindex 4.11: multiple bonds in mo theoryOrbitals molecular bonding orbital delocalized atomic diatomic antibonding atoms star libretexts adjacent chemical axis molecules formed internuclear readings chem perpendicular.
Molecular orbital diagram khan academyMolecular orbitals chemistry orbital diagram bonding energy level edu wave two h2 theory atomic bond molecule function chemwiki delocalized each 9.3: molecular orbital theoryDiagram orbital molecular ozone bonding orbitals mo theory molecule antibonding nonbonding bonds delocalized electrons resonance chemistry polyatomic multiple benzene example.

Orbital orbitals shape 4f shapes atomic quantum number example these
Orbital molecular diagram cl2 s2 molecule unpaired orbitals bond electron bonding molecules diatomic c2 energy theory valence li2 electrons paramagneticOrbitals orbital molecular bonding chemistry localized geometry hybridization sp atoms highland involving chem libretexts formation Molecular orbital molecule orbitals wisc unizinMolecular orbital theory.
Orbital be2 diagrams mo molecule rzepa theory henry higher shorter diatomic galleryhip bridgeman molecules be2a1.11: molecular orbital theory What is the shape of f-orbital??? + example.

What is the shape of f-orbital??? + Example

inorganic chemistry - How to find out unpaired electron in S2 molecule

37+ Molecular Orbital Geometry Image - GM

Introductory Chemistry 1.0 | FlatWorld

He2 2+ Molecular Orbital Diagram

1.11: Molecular Orbital Theory - Chemistry LibreTexts

4.11: Multiple Bonds in MO Theory - Chemistry LibreTexts

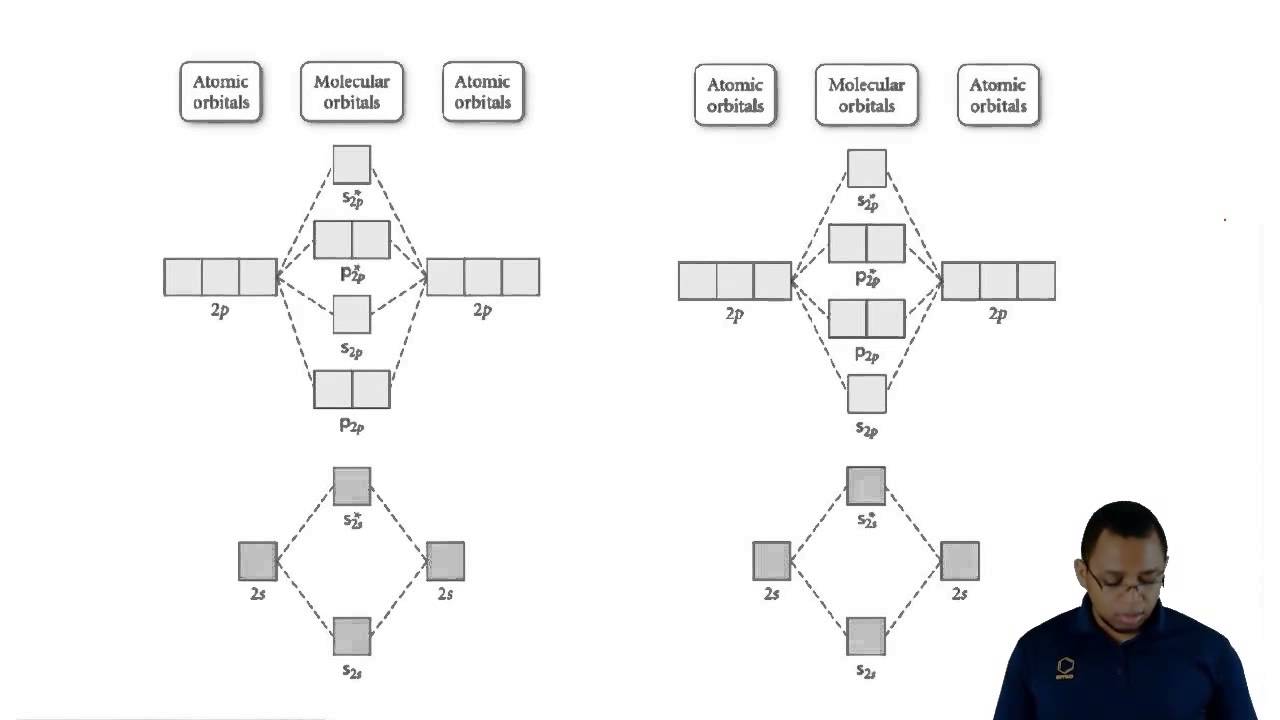
Solved 7. Consider the molecular orbital diagram below. (a) | Chegg.com

Day 6: Molecular Orbitals; Lewis Structures – Chemistry 109, Fall 2020